ONLY FROM THE LEADER IN LASER INTERSTITIAL THERMAL THERAPY (LITT)
MONTERIS DRIVES MINIMALLY INVASIVE BRAIN SURGERY FORWARD WITH THE NEUROBLATE® SYSTEM
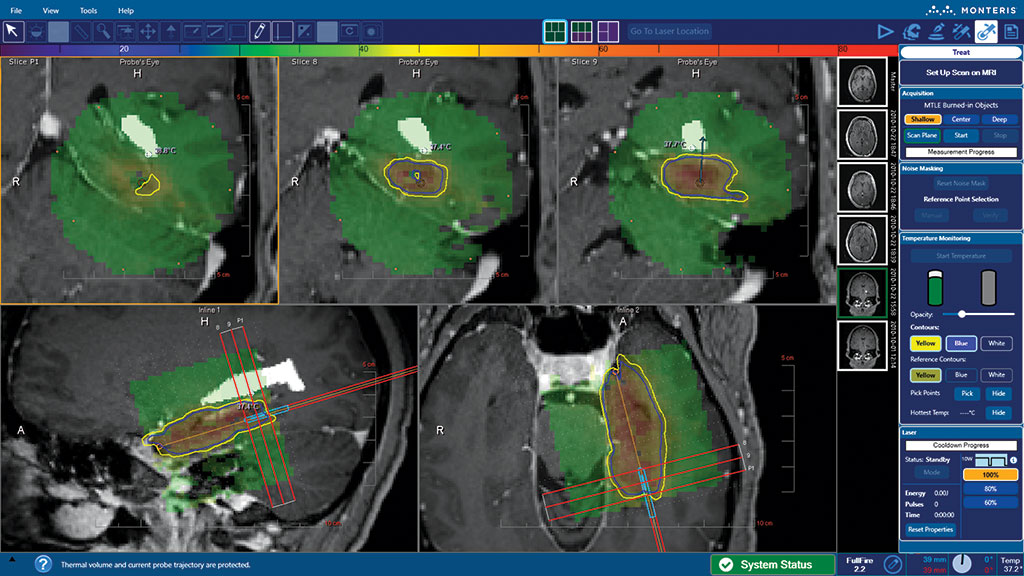
Monteris uniquely offers the most advanced LITT technology designed specifically for use in the brain. NeuroBlate is the only MR-guided robotic laser ablation tool for brain tumors, radiation necrosis, and epileptic foci.
GENERATIONS AHEAD.
NEW TECHNOLOGY & EVIDENCE FROM MONTERIS
LEADING WITH EVIDENCE.
Monteris is uniquely committed to delivering clinical evidence physicians need to make care decisions for their patients and their families. Prospective outcomes for laser interstitial thermal therapy (LITT) are published for brain tumors, radiation necrosis and drug-resistant epilepsy.
NEW! This study of 20 prospective and retrospective patients with meningioma represents the largest cohort of LITT-treated meningioma cases to date. Few treatment options currently exist for meningioma, the most common primary brain tumor. This analysis shows that minimally invasive laser ablation is a viable, safe option with few complications for patients, especially when treatment options are exhausted.

See this important update on evidence for LITT and disruption of the blood brain barrier and blood tumor barrier.

DOWNLOAD A COPY OF A LITT BIBLIOGRAPHY AND CONTACT US TO LEARN MORE ABOUT PROSPECTIVE DATA SPECIFICALLY FOR THE NEUROBLATE SYSTEM.

REQUEST INFORMATION.
Please refer to the Legal Notice for information on Monteris’ privacy policy and use of personal information.
Disclosures
Monteris provides technology for neurosurgeons, which allows them to ablate (destroy with heat), brain structures such as brain tumors, radiation necrosis, and epileptic foci. Monteris technology includes the NeuroBlate System, AtamA, and MiniBolt devices, which may be used together to apply the focused laser energy with little or no effect on surrounding healthy tissue. The NeuroBlate System provides clinicians a tool that offers near real-time control and MRI visualization of the therapy during laser ablation treatment.
All brain surgeries carry risk. Possible adverse events include, but are not limited to, hematoma, embolic events, edema, bleeding, cerebral spinal fluid (CSF) leakage, infection, unintended major tissue damage and permanent neurological deficits. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
Rx Only.