
MONTERIS MEDICAL DISPOSABLES
Delivering Surgical Confidence with Fiber Optic Technology
The NeuroBlate® System includes the NeuroBlate Optic™ Laser Probe, the first and only commercially available laser probe with fiber optic controlled cooling.
Disposables include Optic Laser Probes, Probe Driver, and Monteris Mini-Bolts
FIRST and ONLY commercially available laser probe with fiber optic controlled cooling.
Delivering Surgical Confidence with Fiber Optic Technology
- The NeuroBlate Optic Laser Probe delivers increased confidence for patient safety when in the MRI, as all patient contacting components are non‑metallic.
Freedom to Customize One or More Ablation Trajectories
- Due to its non-metallic fiber optic temperature sensor, the NeuroBlate Optic Laser Probe allows more freedom to customize the trajectory during surgical planning and positioning. This is especially noteworthy for procedures with challenging target locations.
- The NeuroBlate System allows for either single trajectories or multiple, sequential trajectories with one probe. This multiple use of a single probe during a procedure is unique when compared to other brain laser ablation surgery options, thus avoiding additional cost.
NeuroBlate System’s Optic Laser Probes
Fiber optic controlled cooling
- The NeuroBlate Optic Laser Probe includes a sapphire capsule over the laser fiber, enabling prolonged laser firing.
- Pulsed laser firing allows probe cooling between firing intervals, which supports directionality and controlled ablation.
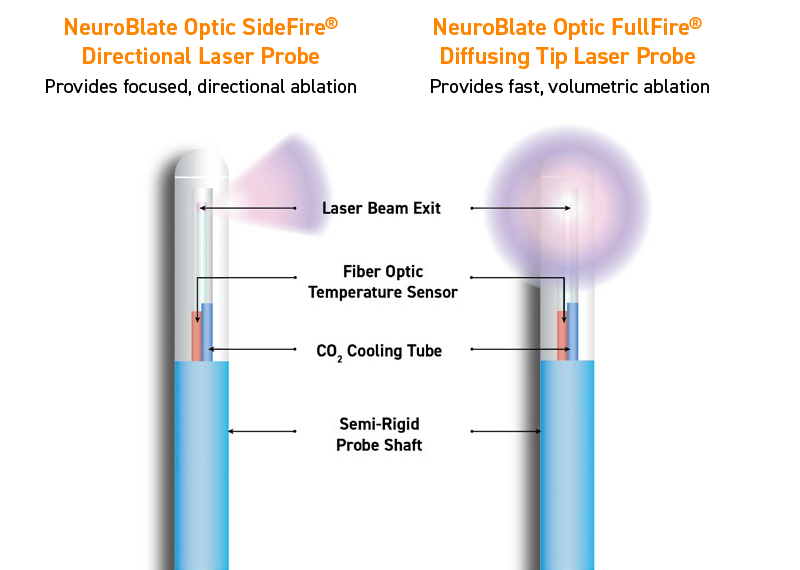
Regulated Probe Cooling
- The fiber optic sensor delivers data to the NeuroBlate Fusion™ Software, which regulates probe cooling and ensures controlled ablation. This provides added safety assurance.
- Pressurized carbon dioxide (CO2) is continuously adjusted by the NeuroBlate Fusion Software to maintain probe tip internal temperature. This helps control tissue ablation.
Optic Laser Probes. Technical Brochure
Disclosures
Monteris provides technology for neurosurgeons, which allows them to ablate (destroy with heat), brain structures such as brain tumors, radiation necrosis, and epileptic foci. Monteris technology includes the NeuroBlate System, AtamA, and MiniBolt devices, which may be used together to apply the focused laser energy with little or no effect on surrounding healthy tissue. The NeuroBlate System provides clinicians a tool that offers near real-time control and MRI visualization of the therapy during laser ablation treatment.
All brain surgeries carry risk. Possible adverse events include, but are not limited to, hematoma, embolic events, edema, bleeding, cerebral spinal fluid (CSF) leakage, infection, unintended major tissue damage and permanent neurological deficits. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
Rx Only.
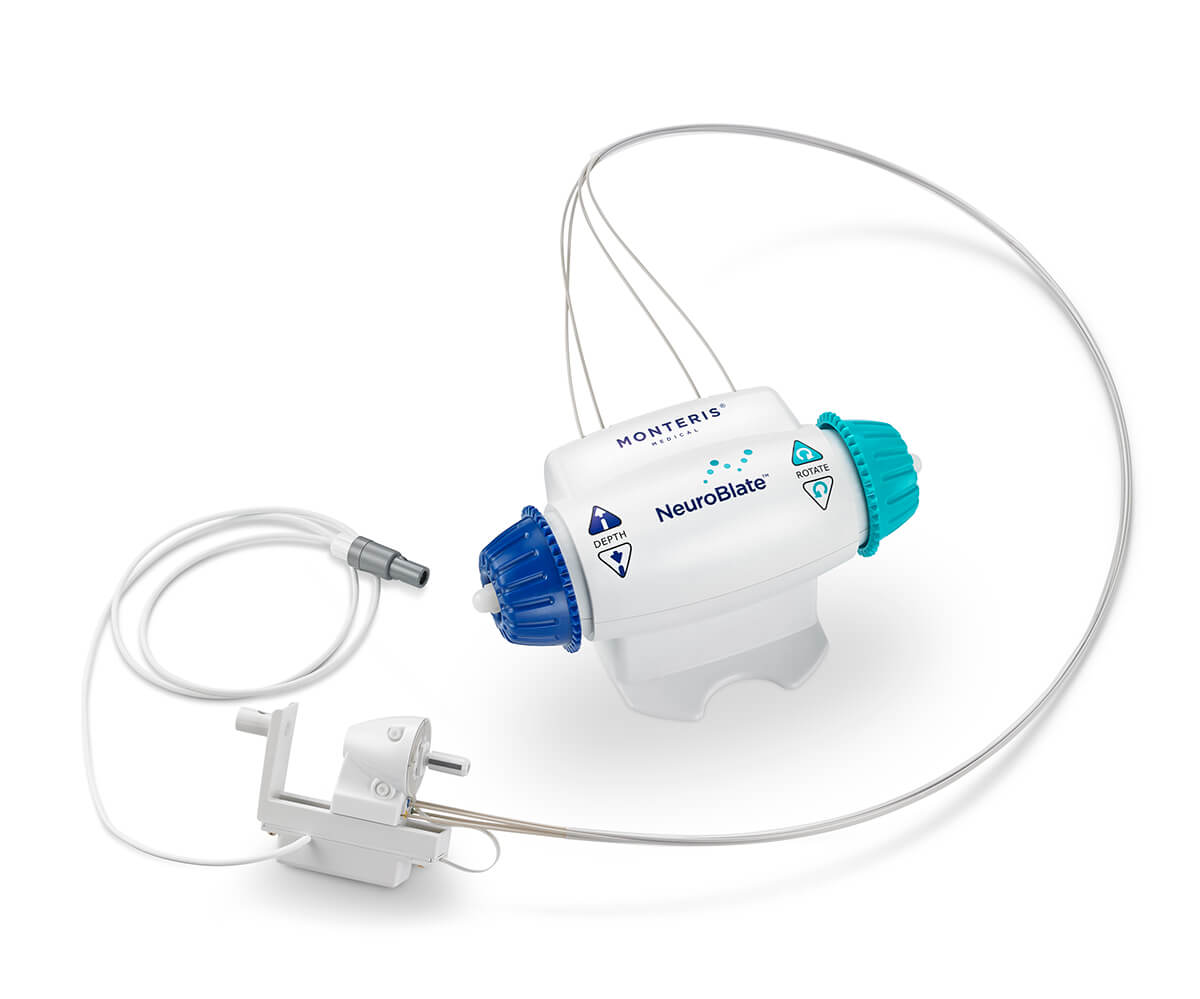
Expanded Cranial Access
The Robotic Probe Driver, designed for use with a skull anchor like the Monteris Mini-Bolt, reduces profile in the MRI for access to virtually any brain location along the ideal trajectory.
NeuroBlate Robotic Probe Driver
Low profile delivery platform for robotic laser thermotherapy
- Precise robotic linear positioning prevents laser probe misplacement.
- Probe can be directed from the work station during the procedure.
- Hands-off laser manipulation eliminates multiple trips into the MR scan room and procedure delays.
Robotic Probe Driver. Technical Brochure
Disclosures
Monteris provides technology for neurosurgeons, which allows them to ablate (destroy with lethal heat) from the inside, brain lesions and brain tumors that may be difficult to approach via traditional methods. Technology includes the NeuroBlate System, AxiiiS, AtamA, and MiniBolt devices, which may be used together to apply the focused laser energy with little or no effect on surrounding healthy tissue. The NeuroBlate System provides clinicians a tool that offers near real-time control and MRI visualization of the therapy during laser ablation treatment.
All brain surgeries carry risk. Possible adverse events include, but are not limited to, hematoma, embolic events, edema, bleeding, cerebral spinal fluid (CSF) leakage, infection, unintended major tissue damage and permanent neurological deficits. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
Rx Only.
Dependable skull fixation for neurosurgical devices
The only cranial fixation bolts with a robotic interface to enable precise laser delivery.
Mini-Bolt VUE™
Cranial bolt with greater visualization of lesion targets near the skull
- Provides the benefits of a minimally invasive option for patients with shallow lesions where open surgery was the preferred option.
- Ablation targets may be optimally defined, conforming to intended ablation boundaries.
- Made from a biocompatible, high mechanical strength ceramic proven to withstand high impact resistance.
- Available in 2.2 mm and 3.3 mm inner diameter.

Mini-Bolt
Cranial bolt system with robotic interface
- Minimally invasive access through a small 4.5 mm diameter hole. Available in 2.2 mm and 3.3 mm inner diameter.
- Enables precise, on-trajectory placement of laser probes.
- Compatible with most stereotactic frames and navigation systems ensuring efficient workflow.

Monteris Mini-Bolt. Technical Brochure
Disclosures
The Mini-Bolt is a disposable device intended to provide placement and skull fixation of neurosurgical instruments or devices with an outer diameter (OD) up to 3.3 mm or 2.2 mm. All brain surgeries carry risk. Possible adverse events include, but are not limited to, tissue injury, infection, and cerebral spinal fluid (CSF) leak. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
Rx Only.